Regulation (EU) 2023/607 addresses imminent risks of medical device shortages in the EU by extending transition timelines of Regulation 2017/745 EU (MDR) up to the end of 2027/2028, thereby allowing continues marketing off legacy devices based on previous MDD/AIMDD Certificates.
However, manufacturers of legacy devices must act now and meet specific conditions set out in the regulation to profit from the longer transition timelines. Most importantly, manufacturers need to lodge a formal and complete MDR application no later than 26 May 2024 and sign a written agreement for conformity assessment with a Notified Body by 26 September 2024.
Navigating the transition of substance-based devices or device-drug combination products (often called borderline products) to compliance with the MDR presents a complex challenge. This is partially due to new or updated classification rules introduced under MDR such as rule 14 and rule 21 (rf. MDCG 2021-24) leading to mandatory up-classification of these device types. Moreover, regulatory uncertainty regarding demarcation from medicinal products was introduced with the new borderline guidance (MDCG 2022-5) by substantially expanding the applicable definitions of pharmacological, immunologic, and metabolic actions.
MDR specifically requires Notified bodies (NBs) to verify device qualification and risk classification as part of their pre-application procedures. Recent statistics published by the European Commission indicate that roughly one fifth of all refused MDR applications are the result of NBs challenging the device status and/or risk class of the respective products (see Figure 1).
Adding to the complexity, capacity of NBs with a suitable scope for borderline products is very limited and resources are expected to further decrease due to the anticipated rush of last- minute applications when getting closer to the May 26 application cut-off timeline.
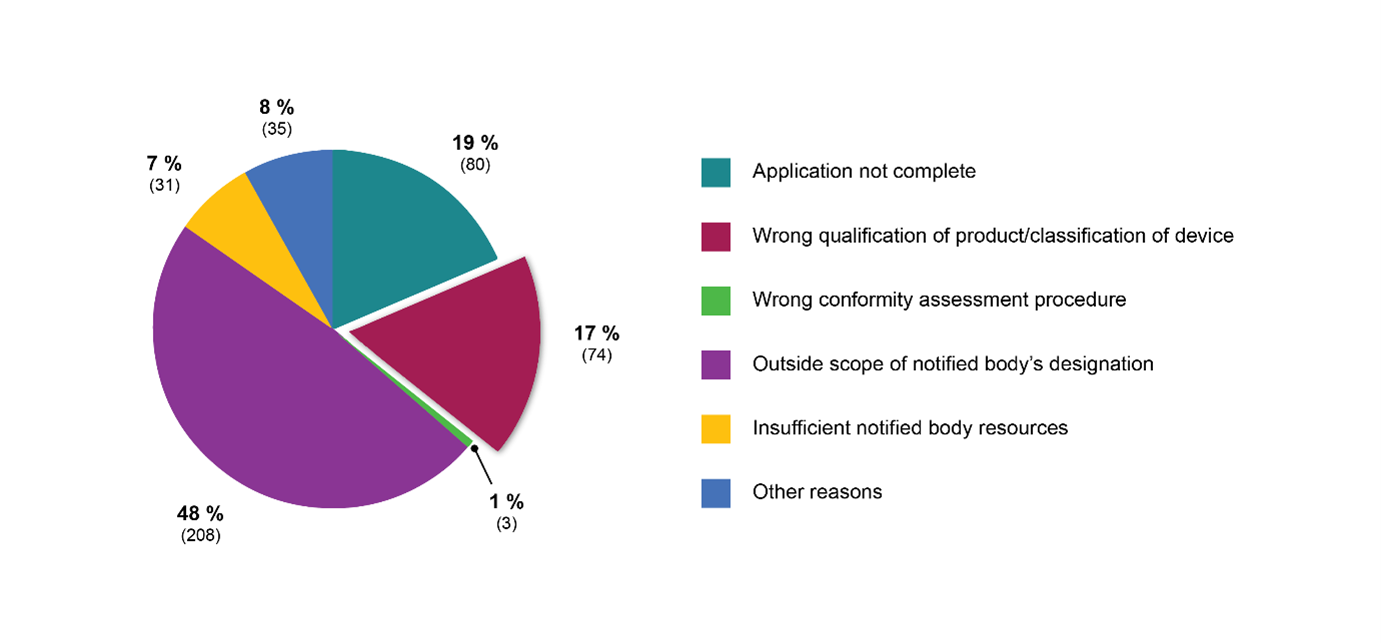
Figure 1: Reasons to refuse applications in number of files (MDR)
Source: Modified from: Austrian National Public Health Institute, Areté, Civic Consulting (2023).*
Leveraging our established relationships with notified bodies and our extensive expertise accumulated over decades as a service provider serving both the pharmaceutical and medical device sectors, we have guided numerous clients through the multifaceted MDR borderline challenges. Therefore, we can support you with:
|
|
Connecting to a suitable notified body with borderline expertise and sufficient resources |
|
|
Lodging a formal MDR application that is considered complete before 26 May 2024 |
|
|
Providing sufficient evidence and documentation to pass a Notified Bodie’s pre-application assessment of device qualification and risk classification |
|
|
Updating the TD of your legacy device to fulfill all MDR requirements |
|
|
Compiling a CTD for ancillary medicinal substances meeting the expectations of Competent Authorities/EMA |
|
|
Managing your conformity assessment and consultation procedure including scientific advice and/or pre-consultation meetings, if required |
|
|
Taking over legal manufacturer responsibility via our subsidiary NEXTEC medical to speed up your MDR certification while you retain full control over sales and marketing of your product. |
* Dashboard for the “Study supporting the monitoring of availability Health Institute (Gesundheit Österreich GmbH / GÖG). Commissioned by the European Commission within the EU4Health Programme (under specific con Executive Agency, implementing framework contract No SANTE/2021/OP/0002). Available at: https://health.ec.europa.eu/medical-devices-topics-interest